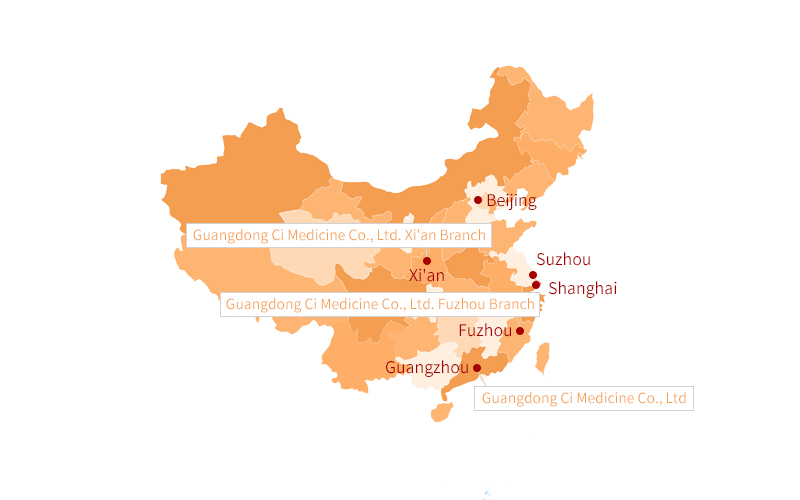
Guangdong Ci Medicine Co., Ltd.
Guangdong Ci Medicine Co., Ltd., originally located at No. 14, Zhenxing Street, Lijiao, Haizhu District, Guangzhou, was moved to 601B, No. 12, Jiangong Road, Tianhe Industrial Park, Guangzhou Hi-Tech Development Zone in 2000.
The company mainly produces and operates the in vivo radioactive drugs; import and export of goods, technology; technical consulting and technical services related to the above items. Its main products are 10 varieties of instantly labelled technetium [99mTc] (1. sodium pertechnetate [99mTc] injection, 2. technetium [99mTc] polymeric albumin injection, 3. technetium [99mTc] dimercaptosuccinate injection, 4. technetium [99mTc] pentetate injection, 5. technetium [99mTc] methylene diphosphonate injection, 6. technetium [99mTc] etifenin injection, 7. technetium [99mTc] phytate injection, 8. technetium [99mTc] methoxyisonitrile injection, 9. technetium [99mTc] biscysteamine injection, 10. technetium [99mTc] biscysteethyl ester injection); operation of sodium iodide [131I] oral solution and fludeoxyglucose [18F]
In 1997, the company obtained the generic drug production license for the ten varieties of instantly labeled technetium [99mTc] drugs and the Drug Production and Operation License, and in July of the same year, it officially supplied the diagnostic and therapeutic radiopharmaceuticals to the Pearl River Delta region. So far, Guangdong Ci Medicine Co., Ltd. has the most approval numbers of instantly labeled technetium [99mTc] drugs in China.
In 2003, the new plant passed the joint inspection of the Certification Center of the National Food and Drug Administration and obtained the Drug GMP Certificate for the first time.
In 2009, the company registered and established Guangdong Ci Medicine Co., Ltd. Xi’an Branch (hereinafter referred to as “Xi’an Branch”) and Guangdong Ci Medicine Co., Ltd. Fuzhou Branch (hereinafter referred to as “Fuzhou Branch”) in Xi’an and Fuzhou. Fuzhou Branch obtained the Drug GMP Certificate in December 2016, and officially supplied the diagnostic and therapeutic radiopharmaceuticals to Fujian Province in March 2017; Xi’an Branch obtained the Drug GMP Certificate in September 2017, and officially supplied the diagnostic and therapeutic radiopharmaceuticals to Shaanxi Province at the same time.











 Industrial Layout
Industrial Layout